The Sky Was the Limit: How Earth’s Ancient Atmosphere Cooked Up Life’s "Impossible" Ingredients
Blog post description.
12/2/20256 min read
The Sky Was the Limit: How Earth’s Ancient Atmosphere Cooked Up Life’s "Impossible" Ingredients
By December 2, 2025
The Paradox of Origins
For nearly a century, the scientific quest to understand the origin of life has been haunted by a single, stubborn "chicken and egg" paradox. We know that life runs on a complex machinery of specific molecules—amino acids to build proteins, lipids to form cell walls, and coenzymes to power metabolism.
The problem is that for decades, we believed the most complex of these ingredients were biogenic—meaning they could only be produced by life itself.
Take sulfur. It is the unsung hero of biology, anchoring the structure of proteins and driving the metabolic engines of the most ancient organisms on Earth. But conventional wisdom held that organic sulfur compounds (organosulfurs) were the exhaust fumes of a living engine. To find them was to find life. If you wanted to make them, you needed a cell.
But if you need a cell to make the ingredients for a cell, how does the first cell ever get built?
This week, that paradox didn't just crack; it shattered.
Groundbreaking research published on December 1 in the Proceedings of the National Academy of Sciences (PNAS) by scientists at the University of Colorado Boulder has revealed that Earth’s early atmosphere was not just a passive blanket of air. It was a chaotic, high-energy chemical factory capable of spontaneously churning out the precise sulfur-based building blocks needed to jumpstart biology.
This discovery doesn't just rewrite the history of Earth. It fundamentally changes the rulebook for hunting aliens on worlds like K2-18b.
The "Prebiotic Kitchen": Simulating the Hadean Sky
To understand the magnitude of this finding, we have to go back 4 billion years to the Hadean and Archean eons. The Earth was a different planet then—a water-world under a faint young sun, with an atmosphere that would be toxic to modern humans.
The prevailing theory has long been that while simple organic molecules (like basic hydrocarbons) could form in this "primordial soup," the specialized, sulfur-heavy molecules were too complex to arise from simple atmospheric chemistry.
Ellie Browne, a chemistry professor at CU Boulder, and her team decided to challenge this. They didn't want to just theorize; they wanted to bake the cake themselves.
The Experiment
The team created a "prebiotic kitchen" in their lab. They filled a chamber with a gas mixture designed to mimic Earth’s early atmosphere. Unlike the famous Miller-Urey experiment of the 1950s—which used electric sparks to simulate lightning in a highly reducing atmosphere—the CU Boulder team focused on photochemistry.
They used a mixture of:
Carbon Dioxide ($CO_2$): The dominant gas of early Earth.
Methane ($CH_4$): A potent greenhouse gas likely present in higher amounts than today.
Hydrogen Sulfide ($H_2S$): The "rotten egg" gas spewed by ancient volcanoes.
Nitrogen ($N_2$): The inert filler of the atmosphere.
They then blasted this mixture with ultraviolet light to simulate the harsh radiation of the young sun, which had no ozone layer to block it.
The Result: A Rain of "Biomarkers"
As the UV light tore through the gases, it triggered a cascade of radical chemistry. Methane and hydrogen sulfide molecules were ripped apart into highly reactive "radical" fragments. These fragments collided and recombined in a frenzied molecular dance.
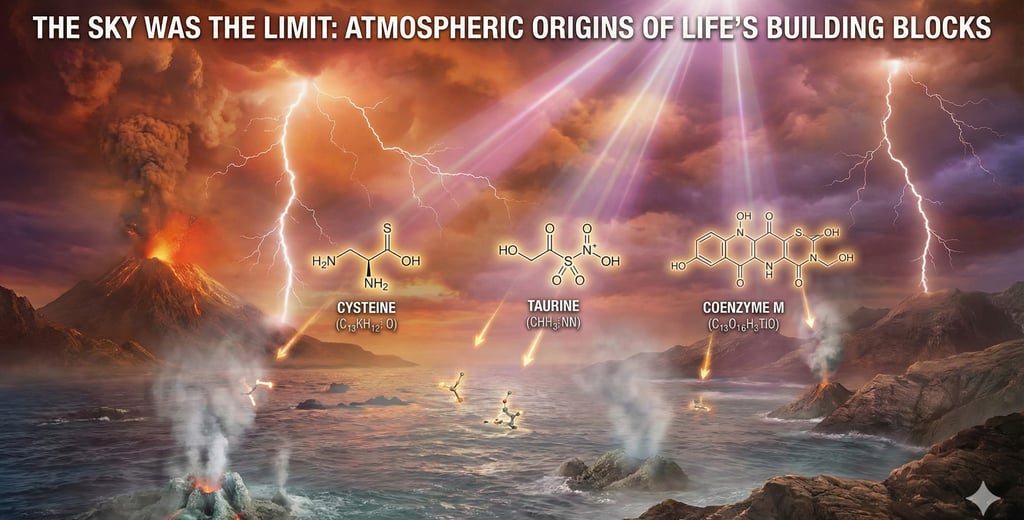
When the researchers analyzed the resulting "goo," they didn't just find random sludge. They found gold—specifically, sulfur-containing biomolecules that biology textbooks insist usually come from living things:
Cysteine
Taurine
Coenzyme M
"We used to think life had to start completely from scratch, but our results suggest some of these more complex molecules were already widespread under non-specialized conditions, which might have made it a little easier for life to get going."
— Ellie Browne, CU Boulder
This means that long before the first microbe swam in the primordial ocean, the sky was literally raining down the spare parts needed to build it.
The "Impossible" Molecules
Why are these three specific molecules so significant? To a chemist/biologist, finding these appearing abiotically is akin to finding a fully formed carburetor in an iron mine.
1. Cysteine: The Architect
Cysteine is one of the 20 standard amino acids that make up the proteins in your body right now. But it’s not just any amino acid; it contains a thiol group ($-SH$).
This thiol group acts like molecular Velcro. When two cysteine molecules in a long protein chain come near each other, their sulfur atoms bond (forming a "disulfide bridge"). This bridge locks the protein into a specific 3D shape. Without cysteine, proteins would be floppy, shapeless noodles incapable of acting as enzymes or structures.
The finding: The study estimates that the early atmosphere could have produced enough cysteine to supply $10^{27}$ (one octillion) cells. That is a staggering surplus of building materials waiting for a contractor.
2. Taurine: The Regulator
You might know taurine from your energy drink can, but in biology, it’s a vital sulfonic acid. Unlike other amino acids, it isn't used to build proteins. Instead, it serves critical roles in metabolism, osmoregulation (balancing salt and water in cells), and membrane stabilization.
Finding taurine suggests that the early atmosphere wasn't just making structural bricks (like cysteine); it was making the regulatory fluids needed to keep a cell stable in harsh, salty ancient oceans.
3. Coenzyme M: The "Smoking Gun" of Ancient Life
This is the most shocking find of the three.
Coenzyme M (2-mercaptoethanesulfonic acid) is not a household name, but it is a celebrity in evolutionary biology. It is found almost exclusively in methanogens—ancient Archaea that produce methane.
Methanogens are widely considered some of the oldest life forms on Earth. Coenzyme M is the specific tool they use to perform the final step of methanogenesis (making methane).
The implication:
The fact that Coenzyme M can be made without life is a paradigm shift.
Old View: Methanogens evolved, and then they painstakingly invented Coenzyme M to help them eat.
New View: The atmosphere produced Coenzyme M naturally. Primitive methanogens evolved to use the tool that was already lying around.
This supports the "scavenger" hypothesis of the origin of life: that early life didn't invent its metabolism from scratch but rather scavenged complex molecules that were already abundant in the environment.
The Astrobiology Crisis: The K2-18b Warning
While this news is a triumph for understanding Earth's past, it is a headache for those looking for life in the present—specifically on other planets.
In recent years, the James Webb Space Telescope (JWST) has turned its golden eye toward K2-18b, a "Hycean" world (hydrogen-rich atmosphere, ocean surface) about 120 light-years away.
The DMS Controversy
JWST detected methane and $CO_2$ on K2-18b, which was exciting. But the data also hinted at the presence of Dimethyl Sulfide (DMS).
On Earth, DMS is produced almost exclusively by marine algae and phytoplankton. It is considered a "robust biosignature." If you see DMS, you scream "Aliens!"
However, the CU Boulder study acts as a massive stop sign.
The same radical chemistry that produced cysteine and Coenzyme M in their lab also produces organosulfur precursors that can look a lot like, or lead to, things like DMS.
If a lifeless atmosphere full of $CO_2$, methane, and sulfur can cook up complex organosulfurs on its own, then detecting those molecules on an exoplanet is no longer a smoking gun for life. It might just mean the planet has a chaotic, sulfur-rich sky.
Nathan Reed, the study's lead author, noted that this finding forces us to be much more careful. We can't just look for a molecule; we have to look for a context that geology alone cannot explain.
Rethinking the "Primordial Soup"
This research fits into a broader movement in science that is redefining the "Primordial Soup" theory.
For a long time, we imagined the "soup" as a quiet pond where chemicals bumped into each other over millions of years. But the new picture of the Hadean Earth is far more dynamic.
The Atmosphere was Active: It wasn't just a blanket; it was a reactor driven by UV radiation.
The Connection was Vertical: We often think of life starting in the ocean. This study suggests the ingredients were made in the sky, then rained down into the ocean.
Imagine a global rain of cysteine and taurine falling into hydrothermal vents.
The vents provide the heat and minerals; the sky provides the complex organic fuel.
This marriage of Sky and Sea might be the true cradle of life.
The "Starter Pack" Theory
Perhaps the most beautiful implication of this study is that the universe seems rigged in favor of life.
If the laws of chemistry dictate that simple gases + light = amino acids and metabolic cofactors, then the "hurdle" for life to begin is lower than we thought. Life doesn't need a miracle. It doesn't need to invent every single screwdriver and hammer in its toolbox.
The universe provides a "Starter Pack."
Planetary formation provides the water and heat.
Atmospheric chemistry provides the amino acids and coenzymes.
Life simply has to provide the spark of organization.
Conclusion: We Are Made of Sky
We often say we are made of "star stuff," referring to the heavy elements forged in supernovae. But this research adds a more local layer to that poetry. The molecules that fold our proteins and power our metabolism may have been forged in the violent, orange skies of our own infant planet.
We are not just children of the Earth's dirt; we are children of its atmosphere.
As we look up at K2-18b and other distant worlds, we must be humble. The chemistry of the cosmos is more creative than we gave it credit for. A planet doesn't need to be alive to cook up the ingredients of life—but knowing that the ingredients are on the menu makes the universe feel a little less lonely.
Inspire
Where vision meets velocity and innovation thrives.
Dream
Reach
contact@skyreachx.com
+1234567890
© 2025. All rights reserved.
